The Healthier Workforce Center of the Midwest (HWC) is pleased to announce two activities designed to promote occupational safety and health research, and to increase public awareness of occupational safety and health concerns:
- The HWC has released a call for pilot research proposals addressing Total Worker Health issues. Awards will be made in one of two categories: Student ($5,000) and New Investigator ($30,000). Proposals are due February 23, 2018. Please see below for details or visit http://HWCPilotGrants.com.
- The HWC announces the Second Annual Occupational Safety and Health Limerick Contest. The winner will receive a “pot of gold” and all the glory that goes with winning. For all the details visit http://OSHLimerickContest.org. Limericks are due by February 28, 2018.

REQUEST FOR PROPOSALS: NEW INVESTIGATOR and STUDENT PILOT PROJECT GRANT PROGRAM
The Healthier Workforce Center of the Midwest (HWC) at the University of Iowa and Washington University is pleased to announce the availability of funds for New Investigator and Student pilot projects.
The HWC is one of six Total Worker Health® (TWH) Centers of Excellence funded through the National Institute for Occupational Safety and Health (NIOSH). The HWC is dedicated to protecting and preserving worker safety and health through knowledge generation and dissemination of evidence-based TWH practices. As a regional Center, the HWC serves the occupational safety and health needs of employees and employers in HHS Federal Region VII (IA, NE, KS, MO). NIOSH defines TWH as “policies, programs and practices that integrate protection from work-related safety and health hazards with promotion of injury and illness prevention efforts to advance worker well-being.” Topics relevant to TWH include improving work organization, assessing the contribution of occupational stressors to the burden of chronic health conditions among employees (e.g., obesity, cardiovascular disease, and depression), optimizing return to work outcomes, and injury/illness prevention strategies populations particularly at-risk for adverse health outcomes (e.g., older/ younger workers, immigrant workers, and those with part time or precarious employment arrangements). Additional resources and information can be found using the following links:
- NIOSH Office for Total Worker Health: https://www.cdc.gov/niosh/twh/
- NIOSH Center for Productive Aging and Work: https://www.cdc.gov/niosh/topics/productiveaging/
- NIOSH Healthy Work Design and Well-being Program: https://www.cdc.gov/niosh/programs/hwd/
Pilot Project Grant Program Objective and Available Funding
The objective of the HWC pilot project program is to encourage development of new and creative research oriented towards prevention/intervention and translation with the strong potential to lead to more comprehensive studies addressing TWH. Awards will cover a one-year period and all funding is conditional of the availability of funds to the HWC. Two types of pilot projects will be considered under this Request for Proposals:
- New Investigator Awards: The HWC intends to fund at least one (1) new investigator award. New investigators may include junior faculty, post-doctoral trainees, medical residents and fellows, doctoral students, scientific staff, and senior faculty newly interested in TWH. If the principal investigator is a student or trainee, a faculty sponsor must be identified.
- Student Research Awards: The HWC intends to fund up to two (2) student research awards. Undergraduate students, graduate students, medical residents, and fellows from the health sciences, engineering, or other applicable programs are encouraged to apply. Each student research proposal must identify a faculty sponsor.
New Investigator and Student projects should have a specific scientific hypothesis and should aim to advance the state of scientific knowledge relevant to TWH.
Eligibility
All persons with interest in TWH, affiliated with an “eligible institution,” and possessing appropriate knowledge, skills, and resources are invited to prepare an application. “Eligible institutions” include 1) for-profit, non-profit, and public or private institutions, 2) units of local or state government and eligible federal agencies, 3) units of local and state tribal government, and 4) faith- or community-based organizations. As a regional Center, the HWC serves the occupational safety and health needs of employees and employers in HHS Federal Region VII (IA, NE, KS, MO). Projects from outside HHS Federal Region VII will be considered to the extent that they address the needs of employers and employees within this region.
Questions about eligibility should be directed to Dr. Kevin Kelly (kevin-kelly@uiowa.edu; 319-335-4755).
Funding
Availability of funds for these proposals is conditional on continued funding of the HWC by NIOSH. Funding is available for the usual categories of NIH grant direct costs, such as salary and fringe for research assistants and graduate students, laboratory supplies, equipment, data analysis, and travel associated with executing the research. However, salary and fringe support for faculty is not allowed, and no meeting/conference travel can be covered. Investigators from within the University of Iowa should not include indirect costs (i.e., facilities and administrative costs) in their budgets.
- For New Investigator proposals, the maximum permitted budget is $30,000 (direct plus indirect).
- For Student proposals, the maximum permitted budget is $5,000 (direct plus indirect).
Investigators are strongly encouraged to contact Mindy Sickels-Sterbenz (mindy-sickels@uiowa.edu; 319-335-4411) for guidance in preparing budgets.
Review Process
Pilot project grant applications will be evaluated by a panel of internal and external HWC Advisory Committee members and others with the appropriate expertise. Reviewers follow procedures similar to those used by federal scientific review panels (see http://grants.nih.gov/grants/guide/notice-files/not-od-09-024.html) and consider relevance of the proposed project to TWH, originality, scientific quality of the approach, the potential for future funding, and the appropriateness of the budget. A written critique will be provided to the principal investigator of each pilot grant application, regardless of funding decision. Review criteria for both New Investigator and Student proposal are appended to this Request for Proposals.
Awards
Proposal reviews will be completed and applicants will be notified of funding status within 60 days following the due date for proposals. Each award will be for a maximum duration of 12 months. The work scope of funded projects must be completed and all expenses incurred within 12 months of the release of funds to the applicant organization.
The release of funds to an applicant organization is contingent upon compliance with federal regulations.
Investigators conducting research involving human subjects and/or animals are encouraged to initiate any applicable human subjects (IRB) or animal care review approval or certification processes as soon as possible.
The pilot project PI must submit documentation of IRB approval to before the HWC will release funds. For projects not involving human subjects and/or animals, a statement as such from the IRB is required as documentation. Most IRBs have a “Human Subjects Research Determination” mechanism that can be used for this purpose. Documentation should be submitted to Dr. Kevin Kelly (kevin-kelly@uiowa.edu; 319-335-4755).
In addition, the project proposal must include a Human Subjects Statement. Proposals without a Human Subjects Statement will be considered non-responsive. Detailed instructions for preparing the Human Subjects Statement can be found at: https://grants.nih.gov/grants/how-to-apply-application-guide/forms-d/general/g.400-phs-398-research-plan-form.htm#Human
Planned Enrollment
For projects involving human subjects, the proposal must include the “PHS Inclusion Enrollment Report.” A fillable version of this form can be found at:
https://grants.nih.gov/grants/how-to-apply-application-guide/forms-d/general/g.500-phs-inclusion-enrollment-report.htm
When completing the form, be sure to check “Planned Enrollment” as the enrollment type.
Reporting Requirements
Each funded investigator is required to submit a mid-year progress report and a final report within 30 days of project completion. These reports shall be submitted according to instructions provided by HWC and shall include reporting of work and activities relating to the current (and past) project(s) awarded by HWC to the investigator/eligible institution. Project proposals must include plans to track and report to HWC subsequent results stemming from each pilot project, including but not limited to grants and contracts developed as progeny of the project, students mentored, MS and PhD dissertations generated, presentations and publications emanating from the project, and interdisciplinary collaborations established as a result of HWC-supported work.
Publications, journal articles, presentations, and similar works relating to HWC-supported pilot projects are to include the following statement: “This [describe the project] was supported, in part, by a pilot project grant from the Healthier Workforce Center of the Midwest (HWC) at the University of Iowa and Washington University. The HWC is supported by Cooperative Agreement No. U19OH008868 from the Centers for Disease Control and Prevention (CDC) / National Institute for Occupational Safety and Health (NIOSH). The contents are solely the responsibility of the author(s) and do not necessarily represent the official views of the CDC, NIOSH, or the HWC.”
Application Content
Applications must conform to the format below:
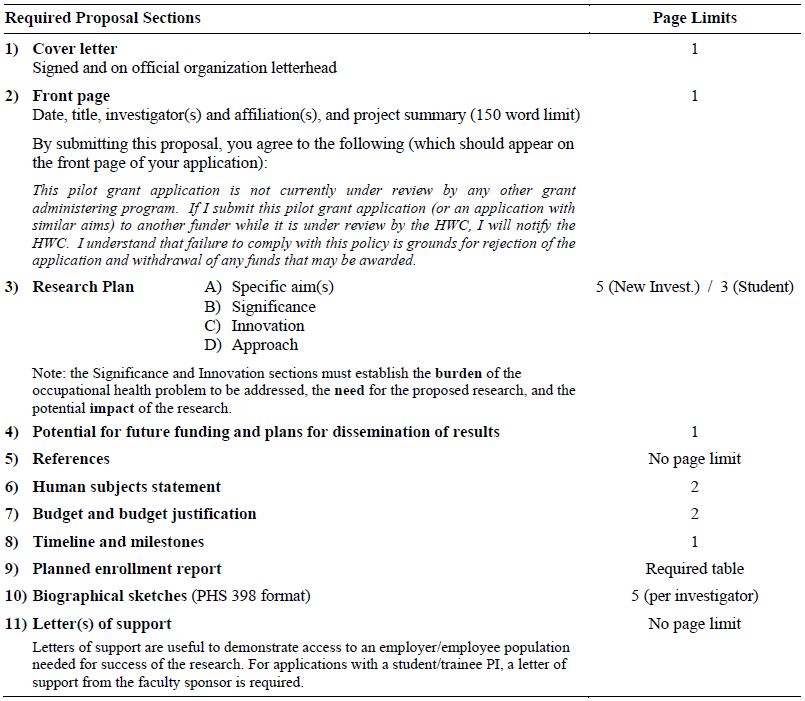
Application Submission Procedures
- Send by electronic mail one copy of the signed cover letter and one complete electronic version (PDF) of the full proposal to Dr. Kevin Kelly (kevin-kelly@uiowa.edu; 319-335-4755). Proposals must be received by the close of business (5PM, Central time) on Friday, February 23, 2018.
- If you cannot submit electronically, send by US mail or private carrier (by Friday, February 23, 2018) a signed original cover letter and one paper copy of the full proposal to:
Dr. Kevin Kelly
University of Iowa
Department of Occupational & Environmental Health
100 Research Park, 104 IREH
Iowa City, IA 52242
Additional Assistance
Applicants are invited to contact Dr. Nathan Fethke (nathan-fethke@uiowa.edu; 319-467-4563) or Dr. Diane Rohlman (diane-rohlman@uiowa.edu; 319-384-4007) regarding scientific questions, and to contact Dr. Kevin Kelly (kevin-kelly@uiowa.edu; 319-335-4755) with questions concerning administrative procedures.
Review Criteria for HWC New Investigator and Student Applications
Significance
- Does the project address an important problem or a critical barrier to progress in the field?
- If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved?
- How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventive interventions that drive this field?
Investigator(s)
- Are the PIs, collaborators, and other researchers well suited to the project?
- If Early Stage or New Investigators, or in the early stages of independent careers, do they have appropriate experience and training?
- If established, have they demonstrated a record of accomplishments that have advanced the field?
Innovation
- Does the application challenge and seek to shift current research or clinical practice paradigms by using novel theoretical concepts, approaches or methodologies, instrumentation, or interventions?
- Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense?
- Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, or interventions proposed?
Approach
- Are the overall strategy, methodology, and analyses methods well-reasoned and appropriate to accomplish the specific aims of the project?
- Are potential problems, alternative strategies, and benchmarks for success presented?
- If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed?
- If the project involves clinical research, are the plans for 1) protection of human subjects from research risks, and 2) inclusion of minorities and members of both sexes/genders, as well as the inclusion of children, justified in terms of the scientific goals and research strategy proposed?
Environment
- Will the scientific environment in which the work will be done contribute to the probability of success?
- Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed?
- Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
Additional Review Considerations
- Is the budget and time to completion justified and reasonable in relation to the proposed project?
- Does the proposal have the potential for future extramural funding?
- Does the proposal promote collaboration of researchers in Federal Region VII?
- Does the investigator have plans to disseminate the results and the continued reporting of related work?